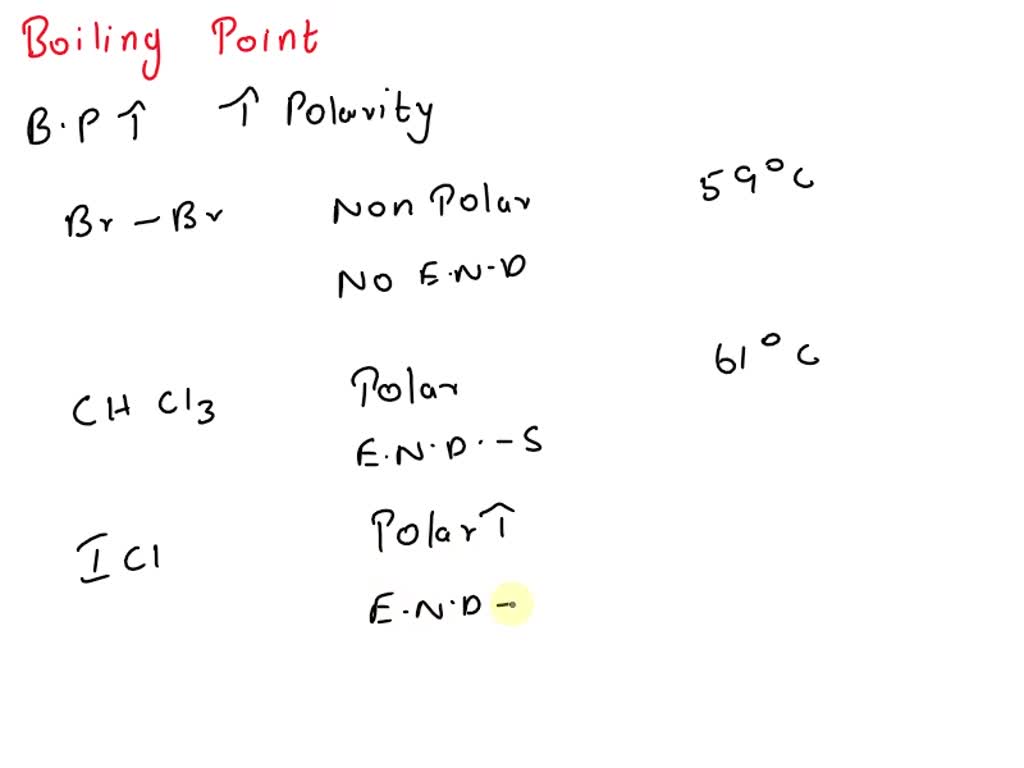
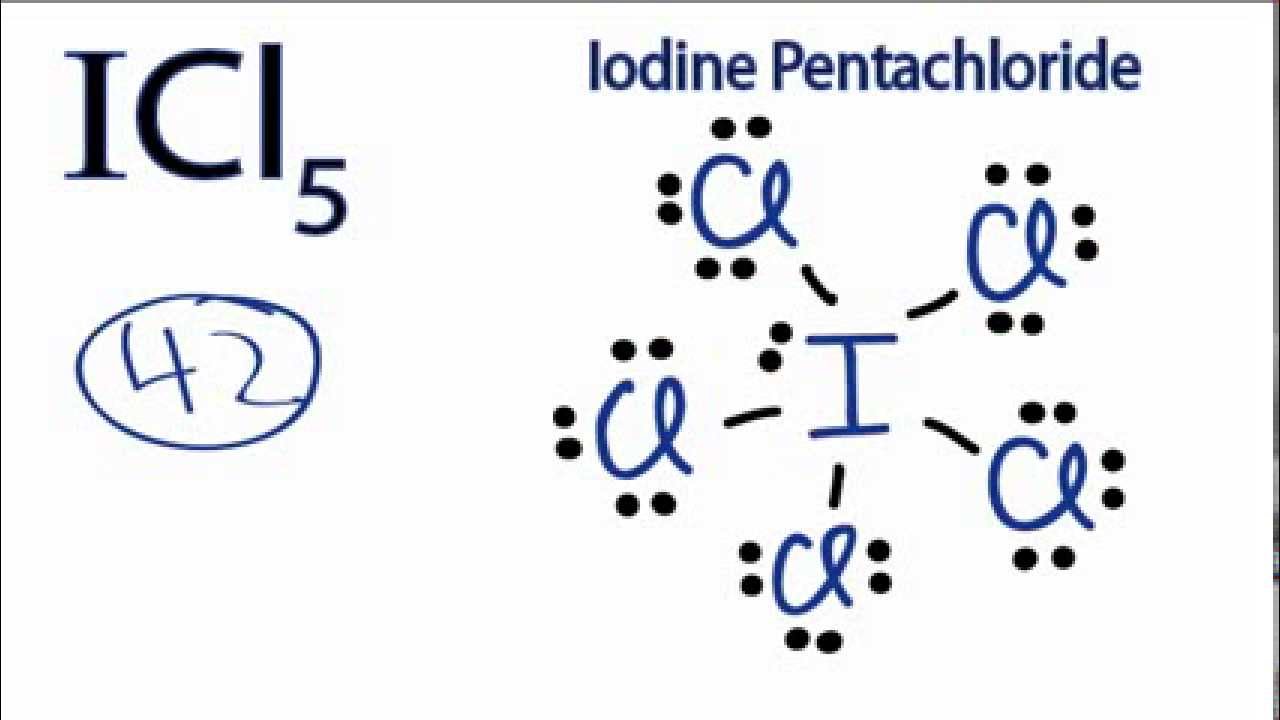
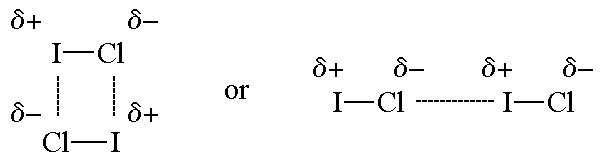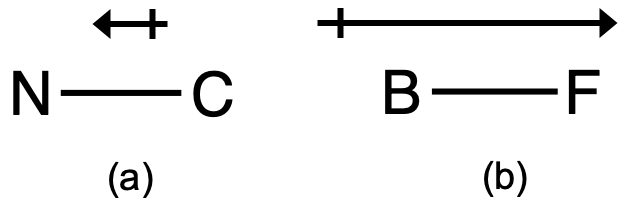![ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download](https://slideplayer.com/10194070/34/images/slide_1.jpg)
ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download

Why is ICl_{2}^{-} a non-polar molecule whereas ICl_{2}^{+} is a polar molecule? | Homework.Study.com

Why is ICl_{2}^{-} a non-polar molecule whereas ICl_{2}^{+} is a polar molecule? | Homework.Study.com

Among the following the total number of polar molecules are. Cl2, OCl, BF3, NO, SO2, XeF4, H2CCl2, OCS .